What is the Most Powerful Covalent Bond: Exploring the Strength of Triple Bonds?
What is the Most Powerful Covalent Bond? Discover the Secret Chemistry
Understanding Covalent Bonds: The Basics You Need to Know
Well, if you’ve ever wondered what makes molecules stick together, it all starts with covalent bonds. So, what exactly is a covalent bond? Essentially, it’s a type of chemical bond where two atoms share electrons. This is super important because these bonds hold atoms together in molecules, whether we’re talking about water, DNA, or even diamonds!
I remember the first time I was introduced to covalent bonds back in high school chemistry class. Honestly, it felt like learning a whole new language. Electrons sharing?! That sounded strange, right? But once it clicked, I realized how fundamental these bonds are in both everyday life and advanced science.
There are two main types of covalent bonds: nonpolar and polar. The nonpolar covalent bond happens when two atoms share electrons equally, like in a molecule of oxygen (O₂). On the other hand, polar covalent bonds happen when the electrons are shared unequally. This creates a slight charge difference across the molecule. Fun stuff, right?
But here's the big question – what’s the most powerful of these bonds? Let’s dig in.
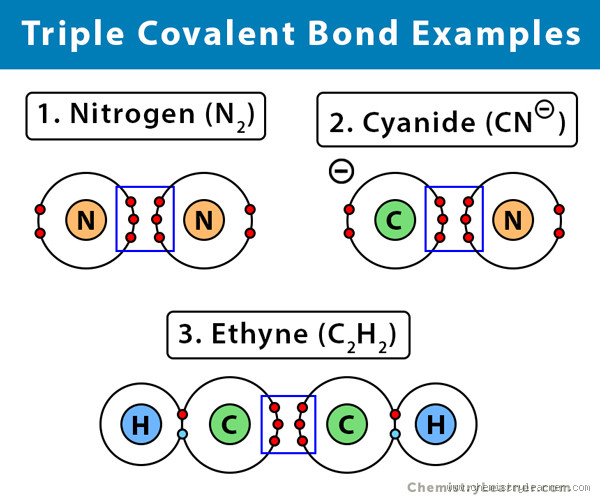
The Most Powerful Covalent Bond: Triple Bonds
What Makes Triple Bonds So Strong?
Okay, so now that we know a little about covalent bonds in general, let’s talk about the most powerful one: the triple bond. A triple bond occurs when two atoms share three pairs of electrons. It sounds simple enough, but the strength of this bond is pretty mind-blowing.
Here’s the thing: more electron sharing means stronger attraction between the atoms. So, a triple bond is way stronger than a single or double bond. For example, take nitrogen (N₂). In its molecular form, nitrogen molecules are held together by a triple covalent bond. This bond is incredibly strong, which is why it takes so much energy to break apart nitrogen molecules. Seriously, breaking that bond is no small feat.
I actually had a conversation with a colleague recently about nitrogen’s triple bond. He’s into biochemistry and mentioned how understanding this bond is crucial for various chemical processes, from industrial applications to even the way plants use nitrogen in the soil. It really made me appreciate just how important these bonds are!
Why Are Triple Bonds So Tough to Break?
This is where it gets interesting: the triple bond is tough because of the sheer number of shared electrons. The more shared electrons, the stronger the bond. So, while a single bond shares just two electrons (one pair), and a double bond shares four, the triple bond shares six electrons (three pairs). This creates a very strong electrostatic force between the atoms, which resists external forces trying to break the bond.
I still remember a chemistry experiment where we had to measure the energy required to break a nitrogen triple bond. Honestly, I was blown away by how much more energy it took compared to breaking single or double bonds. It really hit home how strong these triple bonds are!
Applications of Triple Bonds in Nature and Industry
Triple Bonds in Nature
Did you know that triple bonds play a key role in living organisms? Take DNA, for instance. The nitrogenous bases in DNA molecules are connected by triple bonds between their nitrogen atoms. These bonds are not just strong; they are incredibly precise in their function. Without them, the stability and the structure of DNA wouldn’t be what they are.
In fact, this unique strength is essential for keeping our genetic code intact, and it’s crazy how something so small and powerful can have such a huge impact on life. One of the most surprising things I learned about triple bonds was how they’re responsible for some of the stability in the genetic material. It made me think—how often do we take these tiny chemical wonders for granted?
Triple Bonds in Industry
On the industrial side, triple bonds are involved in some important chemical reactions, especially in the creation of certain chemicals like nitriles. These compounds are used in everything from plastics to pharmaceuticals. There’s a process called the Haber-Bosch process, where triple bonds in nitrogen are broken down to create ammonia, which is crucial for fertilizer production. So, yeah, these powerful bonds are pretty essential for feeding the world!
When I first learned about how industries use nitrogen and its triple bonds, it really opened my eyes to how deeply chemistry affects the world. I mean, it’s wild to think about how something as simple as a covalent bond could play such a big role in agriculture and food production.
Conclusion: Why Triple Bonds Are Chemistry's Heavyweights
Honestly, when you start thinking about covalent bonds, the triple bond is like the heavyweight champion of the bond world. It’s got strength, stability, and it’s vital in both nature and industry. The next time you’re struggling to understand a concept in chemistry or wondering why nitrogen is such a big deal, just remember that it’s all about those powerful triple bonds holding everything together.
Looking back, I have to admit, I underestimated how much there is to learn about bonds. I used to think they were just little connections between atoms, but now I see them as the foundations of everything around us. So, the next time you're marveling at how molecules behave, just know that those triple covalent bonds are likely the ones making it all happen!
How much height should a boy have to look attractive?
Well, fellas, worry no more, because a new study has revealed 5ft 8in is the ideal height for a man. Dating app Badoo has revealed the most right-swiped heights based on their users aged 18 to 30.
Is 172 cm good for a man?
Yes it is. Average height of male in India is 166.3 cm (i.e. 5 ft 5.5 inches) while for female it is 152.6 cm (i.e. 5 ft) approximately. So, as far as your question is concerned, aforesaid height is above average in both cases.
Is 165 cm normal for a 15 year old?
The predicted height for a female, based on your parents heights, is 155 to 165cm. Most 15 year old girls are nearly done growing. I was too. It's a very normal height for a girl.
Is 160 cm too tall for a 12 year old?
How Tall Should a 12 Year Old Be? We can only speak to national average heights here in North America, whereby, a 12 year old girl would be between 137 cm to 162 cm tall (4-1/2 to 5-1/3 feet). A 12 year old boy should be between 137 cm to 160 cm tall (4-1/2 to 5-1/4 feet).
How tall is a average 15 year old?
Average Height to Weight for Teenage Boys - 13 to 20 Years
| Male Teens: 13 - 20 Years) | ||
|---|---|---|
| 14 Years | 112.0 lb. (50.8 kg) | 64.5" (163.8 cm) |
| 15 Years | 123.5 lb. (56.02 kg) | 67.0" (170.1 cm) |
| 16 Years | 134.0 lb. (60.78 kg) | 68.3" (173.4 cm) |
| 17 Years | 142.0 lb. (64.41 kg) | 69.0" (175.2 cm) |
How to get taller at 18?
Staying physically active is even more essential from childhood to grow and improve overall health. But taking it up even in adulthood can help you add a few inches to your height. Strength-building exercises, yoga, jumping rope, and biking all can help to increase your flexibility and grow a few inches taller.
Is 5.7 a good height for a 15 year old boy?
Generally speaking, the average height for 15 year olds girls is 62.9 inches (or 159.7 cm). On the other hand, teen boys at the age of 15 have a much higher average height, which is 67.0 inches (or 170.1 cm).
Can you grow between 16 and 18?
Most girls stop growing taller by age 14 or 15. However, after their early teenage growth spurt, boys continue gaining height at a gradual pace until around 18. Note that some kids will stop growing earlier and others may keep growing a year or two more.
Can you grow 1 cm after 17?
Even with a healthy diet, most people's height won't increase after age 18 to 20. The graph below shows the rate of growth from birth to age 20. As you can see, the growth lines fall to zero between ages 18 and 20 ( 7 , 8 ). The reason why your height stops increasing is your bones, specifically your growth plates.
